Service models
Drug testing platform using iPSC
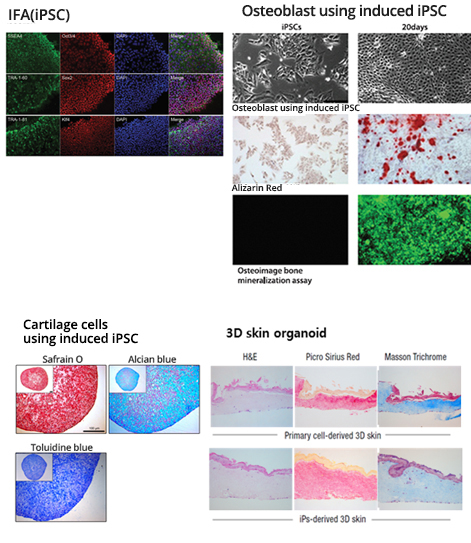
The iPSC-based system replicates patients’ immune systems in a test tube for customized efficacy evaluation. It provides platforms for patient-specific osteoblast, cartilage cells and skin tissues using induced pluripotent stem cells (iPSC) derived from patient’s blood cells. The drug efficacy is tested with in-vitro assay.
| Model | Evaluation interval | Evaluation items |
|---|---|---|
| Osteoblast Cartilage cells |
1~10 days after differentiation (takes 21~30 days) |
|
| Systemic sclerosis (3D skin organoid) | 3 weeks after differentiation (takes 3 weeks) |
|
Service analysis
Patient-specific iPSC
- Generation of iPSC from patient’s PBMC
- iPSC quality control: RT-PCR, IFA
- iPSC banking
Differentiation of iPSC-derived osteoblast and cartilage cells
- Use of iPSC for differentiation into osteoblast/cartilage cells
- Genetic marker analysis of osteoblast/cartilage cells
- Drug treatment to platform of osteoblast/cartilage cells
- Osteoblast: Osteoimage bone mineralization assay
- Cartilage cells: Immunohistochemistry image analysis
- Alzarin red stain and analysis
Systemic sclerosis (3D skin organoid)
- Use of patient’s iPSC for differentiation of fibroblast and keratinocyte/ marker analysis/ production of 3D skin organoid
- Drug treatment to 3D skin organoid
- Analysis of skin morphology, thickness, collagen pattern
- ECM analysis (IHC, IFA, western blot, qRT-PCR)
- Whole 3D skin graft (SCID mice)*